A Whole Blood Culture Assay to Assess Individual Patient Responses to Novel Antiviral Therapeutics
While combination anti-retroviral drugs (cART) can control active HIV-1 virus replication, they are not “curative”. Developing new antiviral therapeutic strategies that can control virus replication, reduce or clear persistent sources of virus infection will require novel diagnostic assays and tools. Such tools must comprehensively evaluate virologic, immunologic and safety parameters for individuals requiring personalized therapies. The J-Test® is a novel assay developed by Jericho Sciences to meet this need.
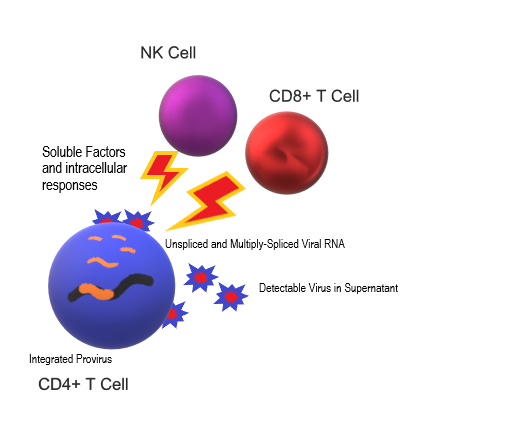
A successful therapeutic approach to reduce persistent sources of virus will likely require:
- selection of clinically safe and effective combinations of therapeutics;
- patient-specific diagnostic testing;
- immune-boosting agents; and
- identification of biomarkers.
Clearing virus infections involves both viral and host interactions.
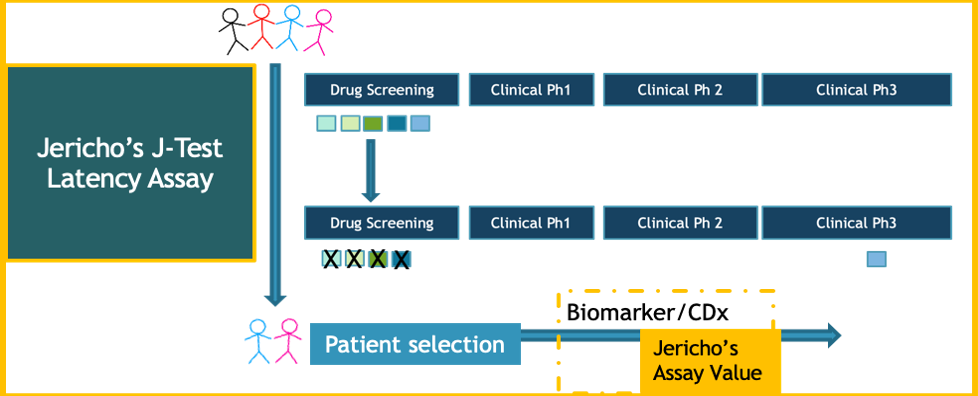
Jericho Sciences’ clinically relevant J-Test® assay supports personalized clinical management of novel therapeutic strategies for HIV-1 infection. Our patented technology assesses patient-specific responses across comparative conditions using the full peripheral blood immunologic compartment. Jericho’s assay reflects the natural physiologic environment of CD4+ T cells, the primary targets of HIV infection.
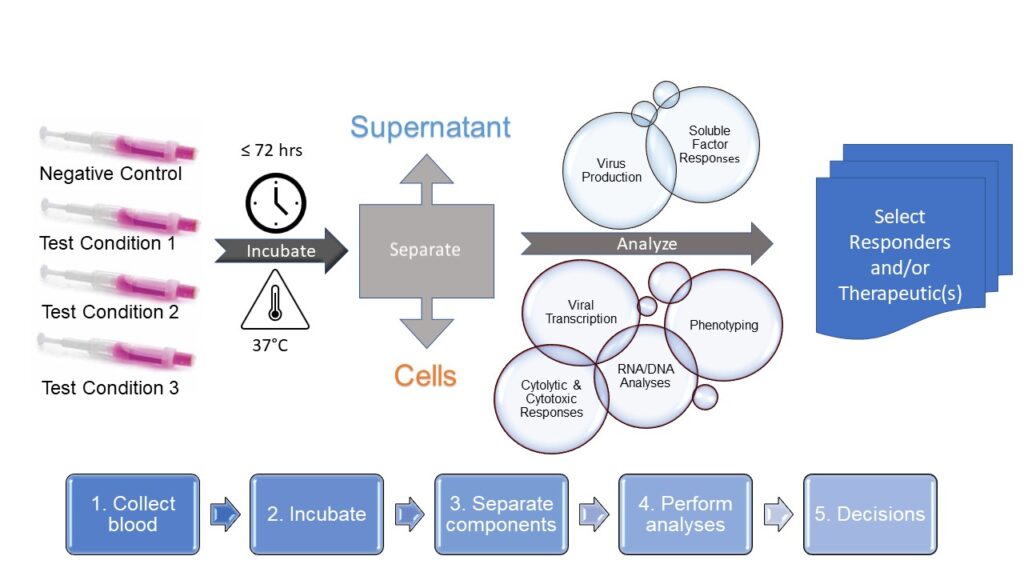
How the J-Test® Works: Whole Blood Culture
- Draw blood into prefilled collection tubes
- Incubate up to 72 hours
- Separate supernatant from cells
- Perform analyses
- Select responders and/or therapeutics
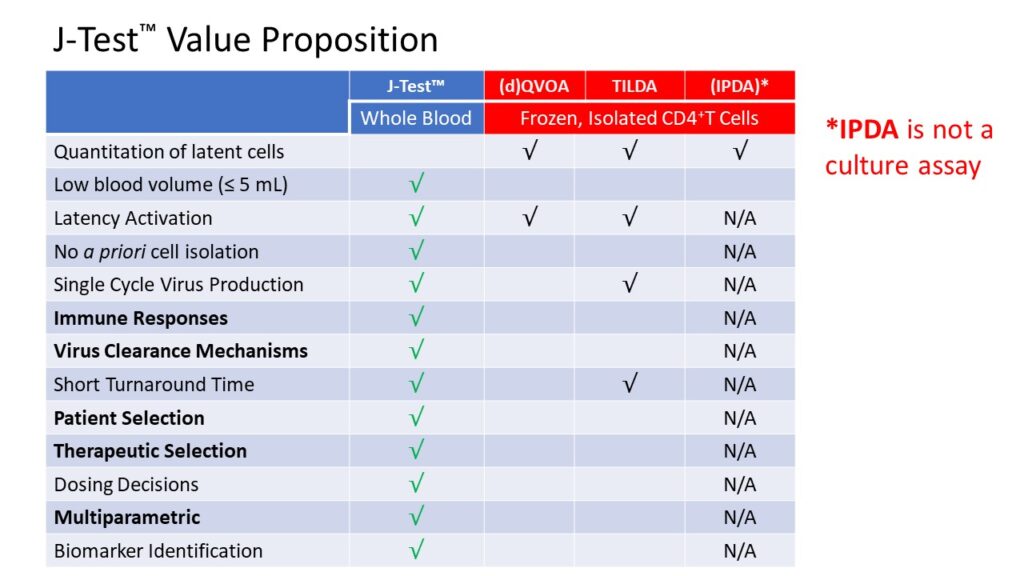
Jericho’s J-Test® Value Proposition
- Clinically Relevant: requires small volumes of fresh blood
- Patient-Specific: internally-referenced
- Short Turnaround Time: results within 1 week
- Multiparametric: viral, cellular and immunologic parameters
- Primary Cells: no prior cell isolation, freezing or manipulation
- Supports Clinical Success: enables identification of biomarkers
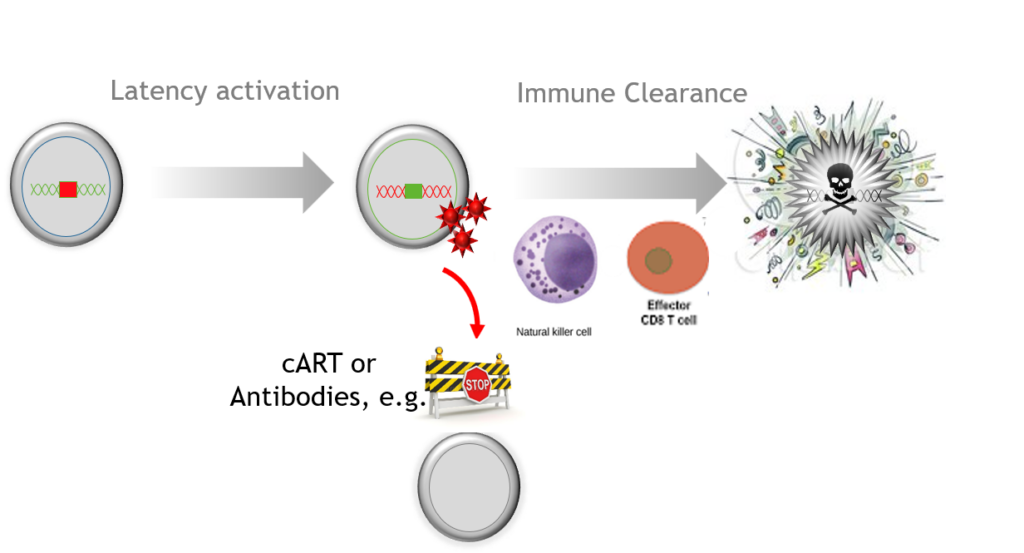
A key remaining question is whether virus-specific immune mechanisms including cytolytic T lymphocytes (CTL) can clear infected cells in ART-treated patients after latency is reversed. […] appropriate boosting of this response may be required for the elimination of the latent reservoir.”
-K Deng et al., (2015) Nature 517,7534: 381-5.
…the heterogeneity of the reservoirs and the multiplicity of the mechanisms which underlie latency and probably vary from one patient to the other and even from one cell to the other in single patient. This observation […] emphasizes the need to evaluate the efficacy of an LRA [latency reversing agent] first ex vivo in cell cultures from a given patient before the administration of this LRA to this given patient in vivo in the context of a clinical trial.”
-G Darcis et al., (2015) PLoS Pathog 11(7)
Contact us
to have Jericho perform the J-Test® on your samples, supporting clinical success of your novel antiviral therapeutics!

Frequency Challenge: How Many Latent Cells in 1 mL of Blood?
| Estimated frequency of latently-infected cells (per million resting CD4+ T cells) | Blood volume to obtain 1 latently-infected cell (mL) | Blood volume to obtain 10 latently-infected cells (mL) | Reference |
| 1 | 10 | 100 | Siliciano, 2013 (using QVOA) |
| 24 | 0.4 | 4.0 | Procopio, 2015 (using TILDA) |
| 60 | 0.17 | 1.7 | Ho, 2013 (using sequencing) |
| 50-100 | 0.10-0.20 | 1.0 – 2.0 | Bruner, 2019 (using IPDA) |