
Our ongoing studies indicate that Jericho’s small molecule provides a significant sustained reduction (95-98%) of multiple measures of FIV viral loads in chronically infected cats for at least 9 months with only 8-9 doses of drug. Extensive testing also indicates that the drug has a good safety profile. Reducing FIV viral loads helps protect cats’ immune systems. We continue to develop this promising drug for treating feline retrovirus infections.
Feline Immunodeficiency Virus (FIV)
- FIV in cats targets immune cells in a manner similar to HIV in humans.
- Impaired immune responses result in more secondary infections and increases in comorbidities.
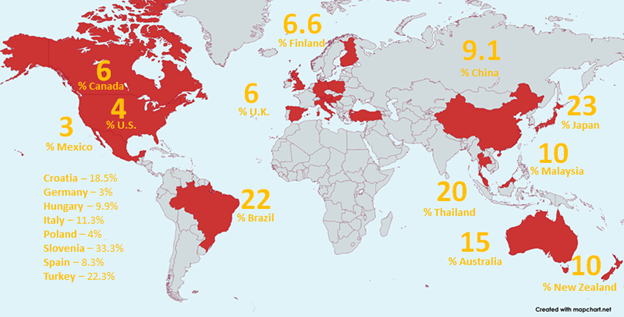
- Approximately 4% of cats in the United States are infected with FIV (ME Westman et al. 2022).
- There are no FDA-approved drugs to treat FIV.
- Current available therapies manage symptoms and may temporarily reduce virus replication.
- Available FIV vaccines can provide some protection.
- There is no evidence that FIV can infect or cause disease in humans.
- There is no cure for FIV.
Worldwide Prevalence of FIV
Feline Leukemia Virus (FeLV)
- FeLV is an immune system-targeted virus
- FeLV significantly increases the risk of developing anemia, leukemia and lymphoma.
- There are no FDA- nor EMA-approved drugs for FeLV.
- Current available therapies manage symptoms, treat secondary infections and manage complications.
- Mean survival is between 1.5‒3 years.
- Vaccines provide some protection against FeLV.
- There is no cure for FeLV.
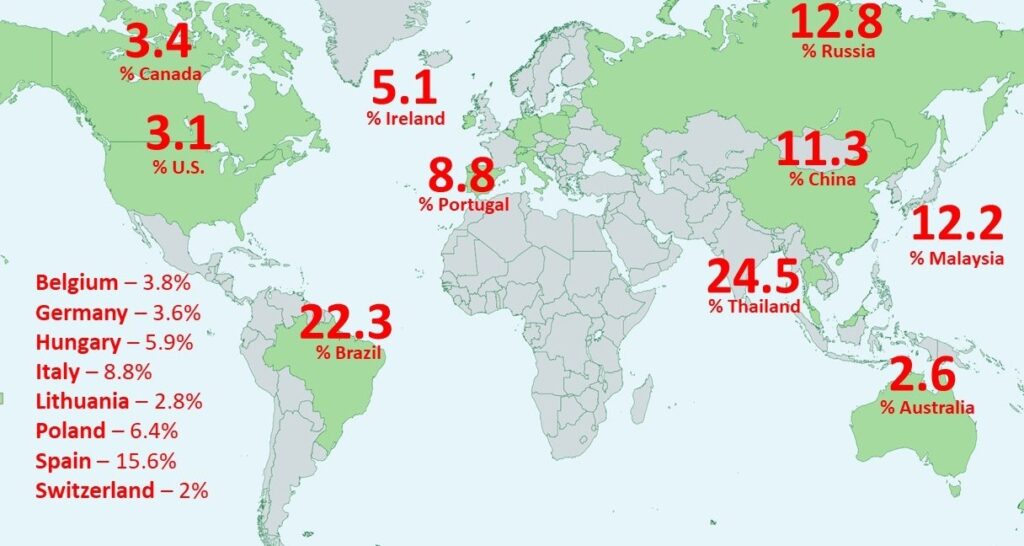
Worldwide Prevalence of FeLV
Estimated Household Cats with Retrovirus Infections
| Country | FIV | FeLV |
|---|---|---|
| United States | 2,960,000 | 2,300,000 |
| United Kingdom | 720,000 | 240,000 |
| Italy | 850,000 | 856,000 |
| Canada | 510,000 | 289,000 |
| Germany | 912,000* | 547,200 |
| Australia | 330,000 | 132,000* |
| New Zealand | 110,000 | 29,000 |
| Japan | 1,680,000 | 438,000* |
| Other EU Countries | 2,680,000 | 2,220,800 |
| TOTAL | 17.8 Million Cats | |